With their extraordinary hardness, brilliance, and durability, diamonds are among the most valuable and rare gemstones in the world. These diamonds typically form in kimberlite pipelines or alluvial deposits over billions of years under intense heat and pressure beneath the earth’s surface. These gemstones are significant because they are widely used in a variety of industries. These diamonds hold great value in the jewelry industry for their elegance and uniqueness.Thus, artisans use them to craft exquisite, high-end pieces of jewelry. The well-known “4 Cs” of a diamond—carat weight, color, clarity, and cut—determine how much it is worth.
These materials find extensive use in the industrial sector due to their distinct physical properties. It includes exceptional durability and thermal conductivity. These materials are used in cutting instruments, polishing abrasives, and as radiators for electronic devices.
Table of Contents:
ToggleWhat Are Diamonds, and How Are They Form?
The Greek word Adamas, meaning unbreakable, originated from the word diamond.
Diamonds consist solely of carbon atoms arranged in a cubic (isometric) configuration, making them the only known mineral composed of just one component, carbon, aside from graphite.
The Earth’s mantle forms diamonds at depths of approximately 140–190 kilometers. The formation of these stones is a complex process that necessitates precise temperatures, pressures, and chemical breakdown conditions. Extreme pressure and heat expose carbon elements to create them. The immense weight of the underlying rock and sediment is typically what causes the high pressure. Whereas the extreme temperature is caused by the internal combustion of the Earth.
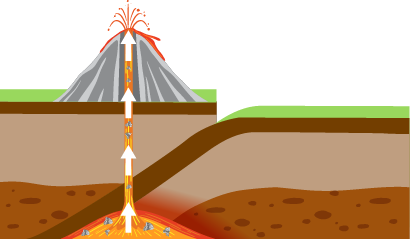
Diamond formation begins when charcoal-rich resources, such as biological material or carbon dioxide, are subjected to high pressure and temperature. It causes the carbon atoms to form crystalline bonds, resulting in the formation of diamond crystals. Via volcanic eruptions, the diamond particles eventually reach the surface of the planet. Volcanic magma transports these gemstones, solidifying into igneous rocks as it cools. These rocks, known as kimberlites or lamproites, possess unpolished diamonds.
In addition to volcanic activity, wear and tear on existing kimberlite pipelines or alluvial deposits can bring diamonds to the surface. These processes gradually expose the diamond-bearing minerals and make them mineable.
In general, the formation of these jewels is a complicated process that takes millions of years and occurs deep within the earth’s mantle of the Earth. These gemstones are some of the most valuable and sought-after gems in the world due to their rarity, attractiveness, and durability.
Fundamental Processes
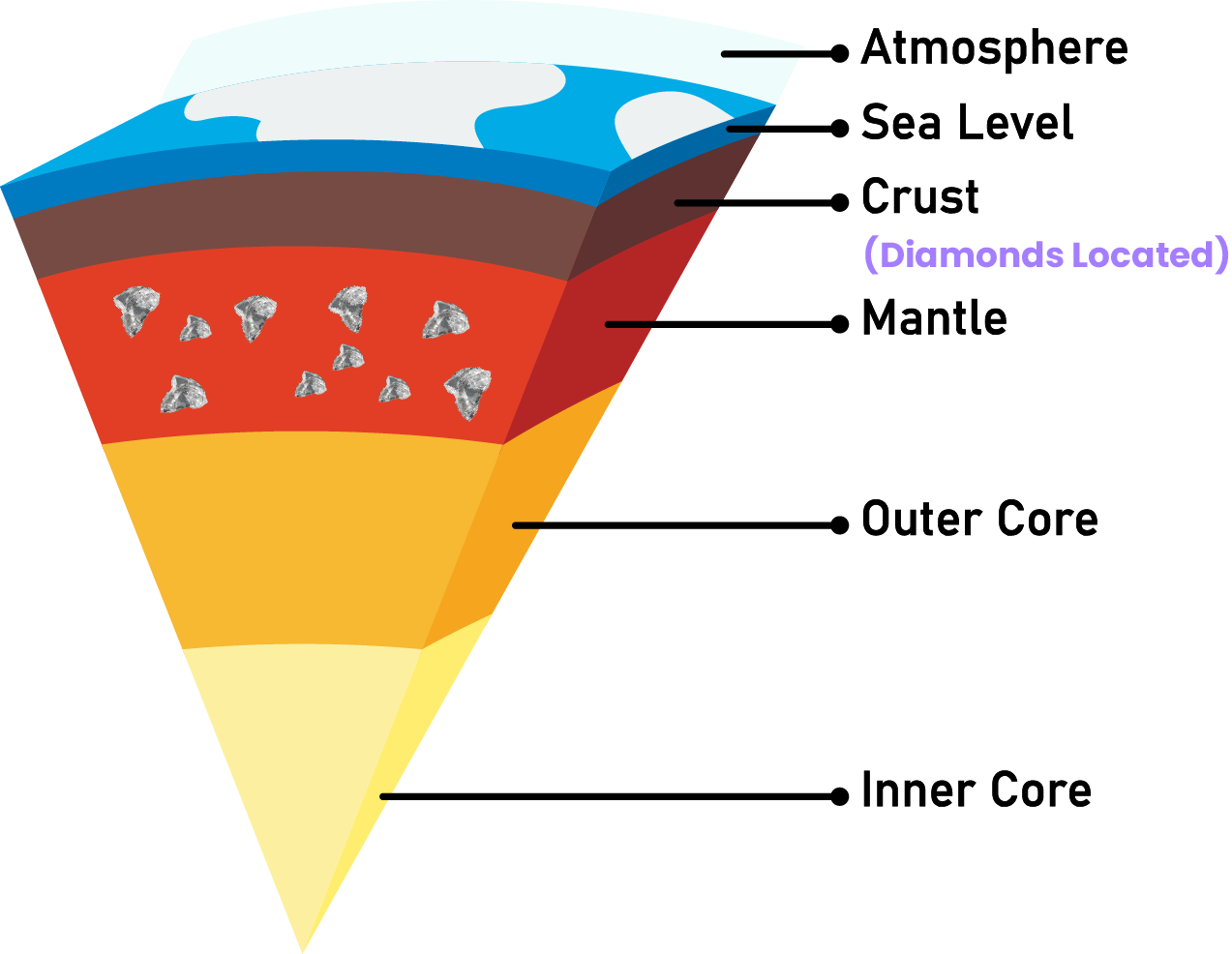
Mantle process: This mechanism is the most widespread method of diamond formation. Approximately 140-190 kilometers beneath the Earth’s surface, these form in the mantle at excessive temperatures and pressures. The high pressure is typically caused by the immense weight of the underlying rock and sediment, whereas the elevated temperature is caused by the internal heat of the Earth. Under such extreme conditions, carbon atoms bond in a crystalline structure to form diamond crystals. These diamond crystals are then conveyed to the surface of the planet by volcanic eruptions, where they are discovered .
Subduction Process: During subduction, one tectonic plate forces another beneath it, recycling the seafloor crust into the Earth’s mantle. During this process, the subducting plate can add carbon-rich debris to the mantle. Under extreme pressure and temperature, this substance can crystallize into diamonds. Volcanic eruptions carry this type of diamond in the form of small crystals within rocks to the surface of the Earth.
Chemical Components of a Diamond
Carbon atoms form a lattice of crystalline structure to create diamond, a naturally occurring mineral. Consequently, it contains a number of distinct chemical properties, such as:
Hardness: The diamond is the most durable known organic substance, with a Mohs hardness scale rating of 10. This implies that nothing other than another diamond can damage or scratch it.
High melting point: Diamond’s extremely high melting point of approximately 3,500 degrees Celsius makes it highly resistant to high temperatures and thermal shock.
Chemical Stability: Extremely chemically stable; it does not react with the majority of compounds involving acids and bases. Thus making it a suitable substance for use in severe or corrosive environments.
Low reactivity: Diamond is a weak electrical and thermal conductor that cannot interact with many other elements and compounds.
Refractivity: Diamond’s elevated refractive index distorts and slows down sight more than the majority of other substances. This characteristic gives these gemstones their characteristic brilliance and sparkle.
Carbon Content: Diamonds consist almost exclusively of carbon, with minor quantities of additional substances such as nitrogen and boron. This high carbon content endows diamonds with distinctive characteristics that make them one of the most expensive and coveted gemstones in the world.
Conclusion
Diamonds are the outcome of incredible geological processes that occur deep beneath the Earth over extremely long periods of time, and their stunning brilliance and appealing looks are the result. Their primarily carbonic chemical composition and distinctive crystal formations provide them with extraordinary attractiveness and durability. These minerals irresistibly appeal to humans, whether mined, lab-grown, or used in industry. Understanding how these gemstones form and the chemicals they contain can help you appreciate their beauty and value.




